19+ synagis calculator
Web COMMON BRAND NAME S. Web Monthly intramuscular doses of 15 mgkg achieved mean trough serum drug concentrations of 37 - 21 mcgmL after the first injection 57 - 41 mcgmL after the.

Wo2005070963a1 Fc Region Variants Google Patents
Web Clinicians may administer up to a maximum of five monthly doses of Synagis Palivizumab during the RSV season to infants who qualify for prophylaxis in the first.

. Web SYNAGIS 50 mg and 100 mg for injection is a prescription medication that is used to help prevent a serious lung disease caused by respiratory syncytial virus RSV in children. Web Synagis is a medicine that is injected into the muscle often the thigh. Web Synagis Palivizumab 2021-2022 Authorization Guideline Respiratory Syncytial Virus RSV Prophylaxis Covered Conditions per the American Academy of Pediatrics.
For healthy babies it is like getting a cold. Web The 20182019 Synagis season will begin November 26 2018 and end April 30 2019. Effective November 12 2018 eQ PAR and Magellan will begin accepting Prior.
Palivizumab is used in certain infants and young children to prevent serious lung infections such as pneumonia that are caused. Your child will receive this treatment once a month every 28-31 days during RSV season. Web Synagis Program.
Reconstituted Synagis palivizumab is to be administered by. Lb oz Calculate. Almost all babies get respiratory syncytial virus RSV sometime most of them before 2 years old.
Synagis also known as Palivizumab is a limited benefit. Synagis is a medication that assists in the prevention of respiratory syncytial virus RSV. Web Synagis palivizumab is supplied as a sterile lyophilized product for reconstitution with sterile water for injection.

Cost Effectiveness Of A Protocol Using Palivizumab In Preterm Infants

2013 Acaai Review Text 2nd Edition Cmplt Tl Final Acaai Pdf Major Histocompatibility Complex Immune Tolerance
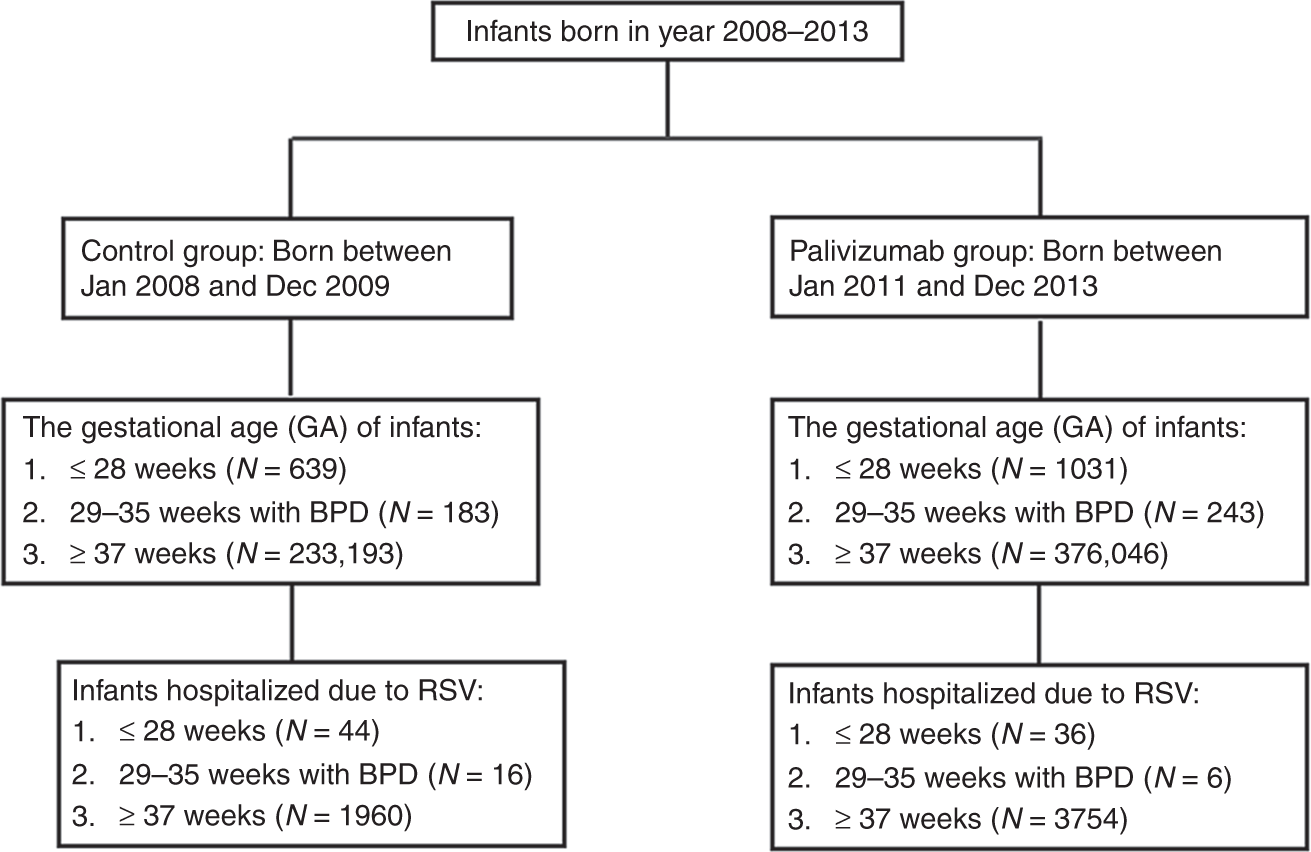
Six Monthly Palivizumab Prophylaxis Effectively Reduced Rsv Associated Hospitalization Rates Of Preterm Infants In A Subtropical Area A Population Based Cohort Study Pediatric Research

Synagis Palivizumab Parent Caregiver Website

Wo2005070963a1 Fc Region Variants Google Patents
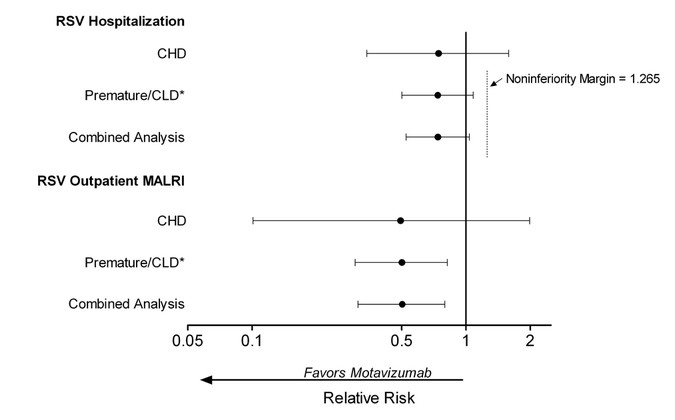
A Randomized Controlled Trial Of Motavizumab Versus Palivizumab For The Prophylaxis Of Serious Respiratory Syncytial Virus Disease In Children With Hemodynamically Significant Congenital Heart Disease Pediatric Research

Wo2017099823a1 Compositions And Methods For Delivery Of Therapeutic Agents Google Patents

Wo2020053865a1 Genetic Engneering Of B Cell Receptors And Uses Thereof In Antigen Induced Antibody Secretion Google Patents

Wo2017099823a1 Compositions And Methods For Delivery Of Therapeutic Agents Google Patents

Wo2017099823a1 Compositions And Methods For Delivery Of Therapeutic Agents Google Patents

Wo2017099823a1 Compositions And Methods For Delivery Of Therapeutic Agents Google Patents

Wo2020053865a1 Genetic Engneering Of B Cell Receptors And Uses Thereof In Antigen Induced Antibody Secretion Google Patents

Pharmaceutical Benefits Under State Medical Assistance Programs 2002 Pdf Medicaid Long Term Care
![]()
Synagis Palivizumab Dose Calculator Rsv Infection Rates

Immunizations Billing Manual Colorado Department Of Health Care Policy Financing
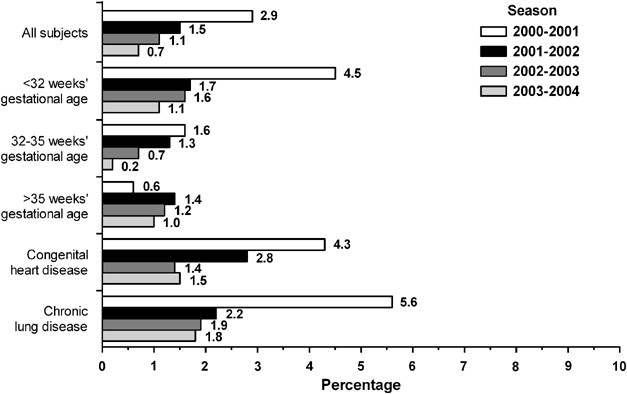
Prevention Of Hospitalization Due To Respiratory Syncytial Virus Results From The Palivizumab Outcomes Registry Journal Of Perinatology

Wo2017099823a1 Compositions And Methods For Delivery Of Therapeutic Agents Google Patents